How Many Electrons Can the S Orbital Hold
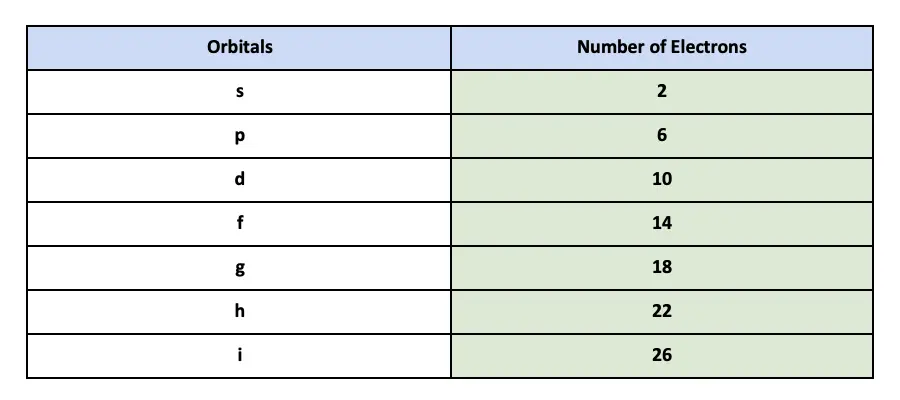
S subshellp subshelld subshellf subshell Table 1. In every sublevel the s orbital can always hold a maximum number of 2 electrons.

Electron Orbitals Shapes Subshells Names Video Lesson Transcript Study Com
One spin-up and one spin-down.

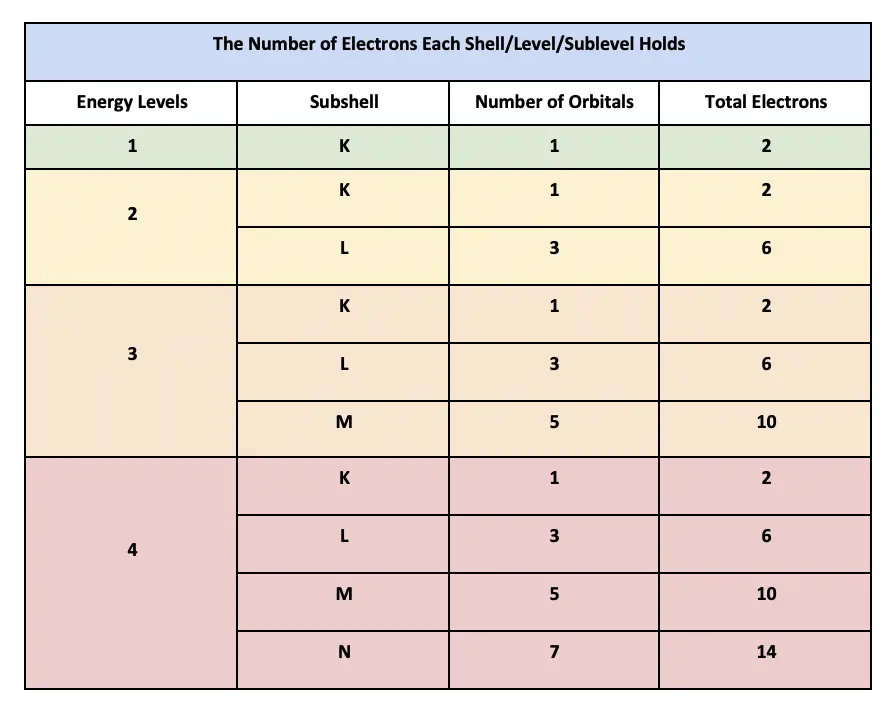
. 1s orbital 1s is the closest orbital to the nucleus and it can hold up to two electrons. What is the identity of each of the three orbitals below. Each shell can contain only a fixed number of electrons up to two electrons can hold the first shell up to eight 2 6 electrons can hold the second shell up to 18 2 6 10 can hold the third shell and so on.
Which of the following types of orbitals can hold a maximum of 10 electrons when filled S. The s orbital is equivalent to the electron shell of the Bohrs atom model. This means that the first shell can hold 2 electrons.
Electrons that occur together in an orbital. The first shell of all atoms has 1 subshell of s-orbitals containing 1 s orbital. How many orbitals are in the sub level f.
Each orbital can hold a maximum of two electrons with opposite spin. The electrons in the S orbital have two different spins which have values of -12 and 12. Which type of orbital is shown in the image right eg.
The s sublevel has just one orbital so can contain 2 electrons max. An s orbital has a spherical shape and can hold two electrons. Show transcribed image text Expert Answer.
5 orbitals 10 electrons. How many p electrons can the third energy level hold. This means that the 1s 2s 3s 4s etc can each hold two electrons because they each have only one orbital.
Each orbital can hold up to two electrons meaning that the 1s 2s 3s 4s and 5s can hold two electrons. 6 electrons can be present. Each of the d sublevels can hold 10 electrons.
The p sublevel has 3 orbitals so max. 3 orbitals 6 electrons. 1 How many electrons can be in each shell.
Electronic orbitals are regions within the atom in which electrons have the highest probability of being found. Who are the experts. Be sure to use the appropriate letter for the orbital e8 5 P d f along with the correct orientation.
Electrons in this orbital are called s electrons and have the lowest energy of any electrons in that principal energy level. An atom is composed of a nucleus containing neutrons and protons with electrons dispersed throughout the remaining space. Thus the third level holds a maximum of 18 electrons.
2s orbital The 2s orbital can hold a maximum of eight electrons. Any orbital holds only 2. The s subshell has 1 orbital that can hold up to 2 electrons the p subshell has 3 orbitals that can hold up to 6 electrons the d subshell has 5 orbitals that hold up to 10 electrons and the f subshell has 7 orbitals with 14 electrons.
See the answer See the answer See the answer done loading. Sp d f and how many electrons can this orbital hold. How many electrons can an s orbital hold.
How many electrons can fit into one orbital. And the 4 sub-levels have seven orbitals and they can hold max 14 electrons. The second shell has 2 subshells.
Instead they are fixed within electronic orbitals. This problem has been solved. How to distribute the electrons in the orbitals.
Each of the s subshells can only hold 2 electrons. For the p orbital you can have 6 electrons. Each orbital can hold no more than two electrons.
How many electrons can 5s hold. How many electrons can 5f hold. Each of the p subshells can only hold 6 electrons.
Failure and nature of Subshells Visualizing Electron Orbitals. For the s orbital you can have 2 electrons. 1 orbital 2 electrons.
Two electrons can occupy the S orbital. Each orbital can hold two electrons. NOTE the pattern of an increase of 4 additional electrons for each succeeding subshell.
Each orbital can hold two electrons. Hence the third electron must move to the first free position on the second energy level n 2. An atom with this configuration is Hydrogen.
So each s sublevel can have two electrons each p sublevel can hold six electrons etc. Why is the s orbital spherical. 7 orbitals 14 electrons.
See full answer below. The subshells s p d and f contain the following number of orbitals respectively where every orbital can hold up to two electrons maximum. This way that the s orbital can contain increase to 2 electrons the ns orbital can contain up to six electrons the d orbital deserve to contain as much as 10 electrons and the f orbital deserve to contain up to 14 electrons.
Why is 3rd shell 8 or 18. That is from 1s to 7s each of them can only have 2 maximum electrons because each of them has only 1 single s. How many electrons can one p orbital hold.
The f subshell has a total of seven orbitals and each orbital can hold two electrons and so the f subshell can hold a total of 7214 electrons. How many can each orbital hold. The d sublevel has 5 orbitals so max.
What is the angle between the orbitals in sp2 hybridization. The s orbital can hold up to two electrons. 1 s-orbital and 3 p-orbitals.
Electrons however are not simply floating within the atom. Every subshell has a of orbits spdf that can each hold 2 electrons each one has the opposite spin of the other. The five d orbitals can hold up to 10 electrons.
The second energy level has four sublevels a circular 2s orbital and three elliptical orbitals 2p 1 2p 2 2p 3. 2 in the s orbital 6 in the three p orbitals and 10 in the five d orbitals. The s subshell has 1 orbital that can hold up to 2 electrons the p subshell has 3 orbitals that can hold up to 6 electrons the d subshell has 5 orbitals that hold up to 10 electrons and the f subshell has 7 orbitals with 14 electrons.
10 electrons can be present. How many orbitals are in the sub level d.
No comments for "How Many Electrons Can the S Orbital Hold"
Post a Comment